Motor Neuron Disease (MND) Research: Using Stem Cells to Develop Treatments
3 October 2023 | By: Helen Devine | 3 min read
Researchers at Newcastle University are using innovative methods to develop treatments for motor neuron disease (MND).
This rare neurological condition occurs when messages from motor neurones - found in the brain and spinal cord - stop reaching the muscles. Read on to learn how our researchers are using patient stem cells to treat MND.
Contents:
-
What is motor neuron disease?
-
The use of stem cells in MND research
-
Exploring the role of mitochondria
-
The possibility of repurposing drugs
What is motor neuron disease?
Within our bodies’ nervous systems, nerve cells (also known as neurons) are the components which pass messages through the body. The nerve cells responsible for movement are called motor neurons, and they carry the messages from our brains to our muscles, telling them how and when to move.
Motor neuron disease (MND) occurs when the motor nerves stop working properly and eventually die - known as neurodegeneration. This means that the brain can no longer effectively communicate with the muscles which, in turn, become weak and unresponsive. MND affects muscles in the arms and legs, those involved in speech and swallowing, and can eventually impact the muscles required to breathe.
The disease affects around 1 in 50,000 people in the UK and can impact adults of all ages, although the risk of diagnosis increases with age. In the most common type of MND - amyotrophic lateral sclerosis (ALS) – approximately half of patients die within two years of a diagnosis. Recent high-profile cases that highlight the awful consequences of MND are Dodie Weir (who died in December 2022) and Rob Burrow, whose cases have helped spread public awareness of the disease, alongside the need for continued research.
Despite lots of research, scientists are still not sure exactly why motor neurons die and there are currently no effective treatments for motor neuron diseases.
The use of stem cells in MND research
To try to understand why motor neurons fail we need to compare faulty motor neurons with healthy ones. Unfortunately, motor neurons do not regenerate; meaning we cannot take a sample of a patient’s motor neurons to study them in a dish. Instead, we can take a sample of skin cells from patients with MND and then, using a Nobel-prize winning method called reprogramming, we’re able to turn them into stem cells.
Stem cells are cells that have the potential to become any other type of cell. We can grow stem cells in a dish until there are thousands of them, which can then be turned into motor neurons by giving them chemical cues over a rough 30-day period; at the end of this period, motor neurons are created in the dish. We can use these motor neurons to understand the disease process and to test potential new treatments for MND.
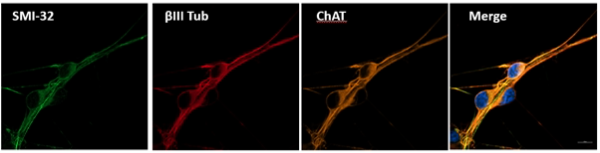
In this picture, we can see motor neurons which have been made from stem cells in the lab. The SMI-32 in green and the ChAT in orange are both markers of motor neurons, and the red beta-III tubulin shows up in nerve cells. In the final image, all three markers are joined together with blue for the cell nucleus to show motor neurons which can then be used for research.
Exploring the role of mitochondria
Researchers Dr Helen Devine and Adam Creigh, based in Newcastle University’s Wellcome Centre for Mitochondrial Diseases, are investigating whether there is a problem with the junction between the motor nerve and the muscle in MND called the neuromuscular junction (NMJ).
The team has made “NMJs in a dish” for the first time in Newcastle and is developing this technique further to separate the motor nerves from the muscle cells, allowing researchers to look at each part of the NMJ separately. Examining the difference between healthy cells and MND cells using highly specialised imaging and electrical tests will determine if there is a potential target for drug therapies. One possible target is the mitochondria.
Most of the energy in cells comes from highly specialised energy powerhouses called mitochondria. Motor nerves need a lot of energy and many studies of MND have found that there is a problem with the mitochondria in MND. The NMJ in particular needs a lot of energy to turn the electrical message from the nerve into a chemical message that travels to the muscle (as well as recycling these chemical signals) - meaning they are therefore likely to be particularly affected if there is a problem with the mitochondria.
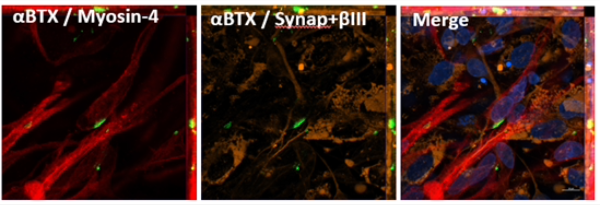
In this picture, green is used to show alpha-bungarotoxin which is found on the muscle side of a neuromuscular junction; the red staining shows the muscle and the orange staining is used to show the nerve. These images are joined together in the final picture to show neuromuscular junctions grown from stem cells in Newcastle for the first time.
The possibility of repurposing drugs
Drug therapies that slow, stop or reverse motor neuron loss are essential for the treatment of patients with MND. However, developing an experimental drug from a laboratory can take 10-15 years before it reaches patients - a period of time MND patients simply do not have.
Repurposing drugs means using drugs that are already used to treat other diseases. This has advantages over traditional drug development as the drugs have already been proven to be safe in humans, which therefore reduces the costs and improves the efficiency of drug discovery.
The team are testing repurposed drugs on the motor neurons of patients with MND to see if they can identify treatments that stop the motor nerves from dying in the dish. Promising drugs from these drug screens will be considered for clinical trials in patients with MND.
You might also like:
- Discover more articles relating to our research in Ageing and Health
- Learn more about our Centres of Research Excellence (NUCoREs)
- Find out more about the MND Association and the latest research on motor neurone disease
- Explore Wellcome Centre for Mitochondrial Research and the work we’re doing there
.
