Human Skin Atlas: the molecular 'recipe' for scarless healing
3 February 2025 | By: Newcastle University | 3 min read
Can the project described as 'a Google Maps for the human body' transform regenerative medicine and skin transplants?
For the first time ever, researchers from Newcastle University and the Wellcome Sanger Institute have created a single-cell atlas of human prenatal skin to understand how skin forms and can heal without scarring. The findings from this study could be transformative for burn victims and people with hair loss. Read on to discover how.
Contents:
- How does skin scar?
- Why build a Human Cell Atlas?
- Mapping the human body
- A 'molecular recipe' for success
How does skin scar?
Scars form when the skin heals following any injury, for example due to an accident, some skin disorders, or surgical wounds.
In normal scarring, a patch of fibrous tissue grows to replace the damaged skin, resulting in the loss of sweat glands and hair follicles.
Unlike adult skin, prenatal human skin heals without scarring – understanding the secrets of prenatal skin could unlock new opportunities for skin healing.
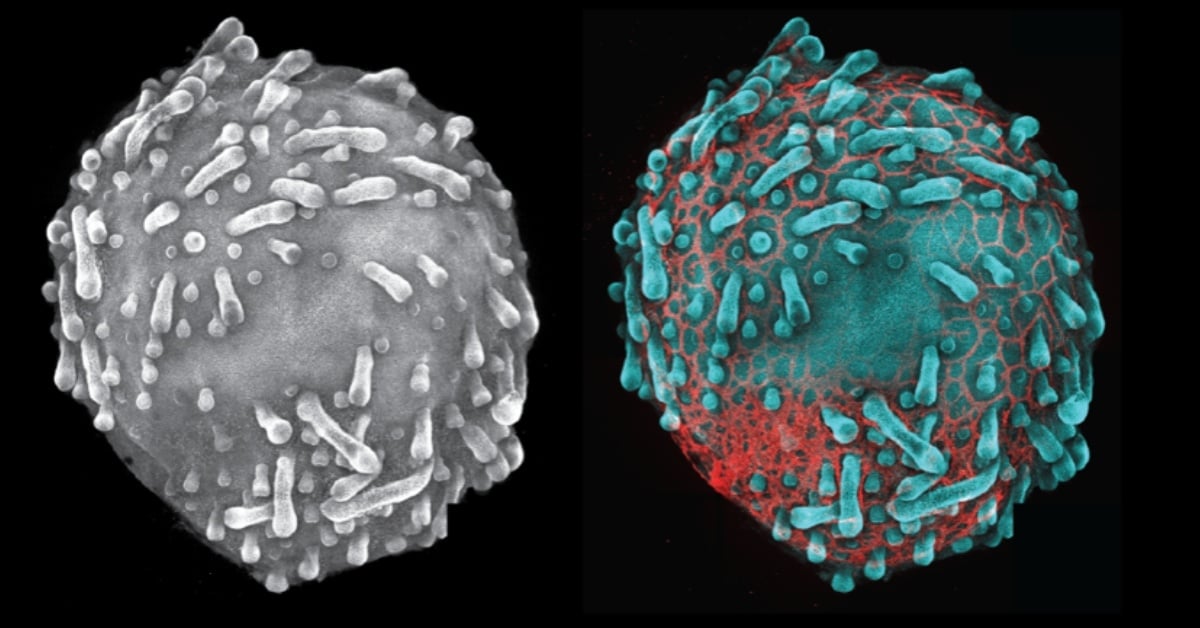
Skin organoid showing hair follicles with endothelial cells (CD31,red). Credit: Karl Koehler
Why build a Human Cell Atlas?
Cells are the building blocks of the human body – approximately 37 trillion cells make up the adult human body. However, we still do not know the different cell types in our body, their precise location, how they maintain health and what goes wrong in disease.
Described as 'a Google Maps for the human body', the Human Cell Atlas (HCA) aims to map all cell types in the healthy body from development to old age to drive major advances in healthcare and medicine worldwide. HCA researchers use cutting-edge single-cell and spatial genomics technologies at scale, combined with powerful computing and AI methods to map the cells in the human body.
Mapping the human body
As part of the Human Cell Atlas, our researchers provided a molecular 'recipe' to build skin – a prenatal skin cell atlas – and showed the value of lab grown skin organoid (derived from pluripotent stem cells) to study congenital skin disorders.
In collaboration with the Wellcome Sanger Institute, our researchers used single-cell sequencing and other genomics techniques to uncover how human skin and hair follicles are formed. The study was authored by Dr Hudaa Gopee, Professor Muzlifah Haniffa, and Dr Elena Winheim among others, and was published in Nature.com.
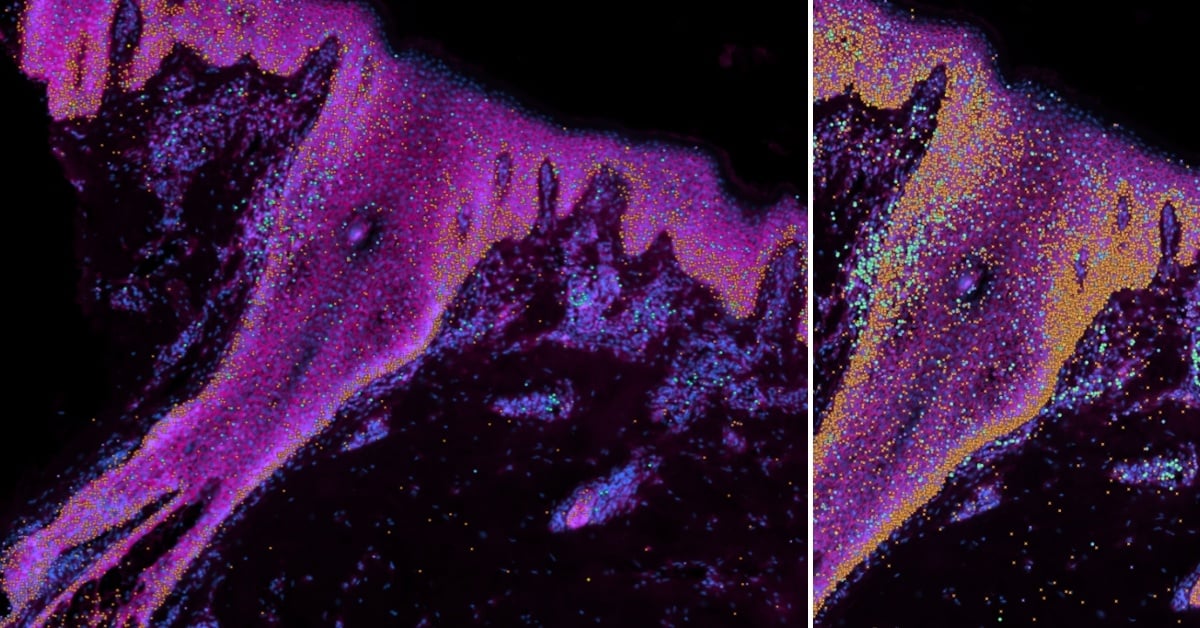
Xenium images of human skin demonstrating some selected genes of interest: cyan - stem cells; orange - keratinocytes; green and purple - immune cells. Credit: Wellcome Sanger Institute.
Our scientists analysed individual prenatal skin cells in space and time, and the cellular changes that regulate skin and hair follicle development using cutting-edge single-cell sequencing and spatial transcriptomics.
Through this, they were able to discern the steps that outline how human hair follicles are formed when compared to mouse hair follicles. The researchers used adult stem cells to create a 'mini organ' of skin in a dish - known as an organoid - with the ability to grow hair. After comparing the molecular characteristics of skin organoids with prenatal skin, they found the skin organoid model more closely resembled prenatal skin than adult skin, and that blood vessels did not form in the skin organoid as well as prenatal skin.
The team added immune cells known as macrophages to the organoid, which promoted the formation of blood vessels within the tissue using 3D imaging. Although it was already known that macrophages protect the skin from infection, this is the first time they have been shown to play a key role in the formation of human prenatal skin by supporting the growth of blood vessels.
A 'molecular recipe' for success
Crosstalk between non-immune and immune cells supports the formation of hair follicles. This makes it crucial for the formation of new blood vessels (angiogenesis) in the skin, and means it can be used in scarless wound healing.
Dr Elena Winheim, co-first author of the study, says: 'With our prenatal human skin atlas, we’ve provided the first molecular ‘recipe’ for making human skin and uncovered how human hair follicles are formed before birth. These insights have amazing clinical potential.'
'Our prenatal human skin atlas and organoid model provide the research community with freely available tools to study congenital skin diseases and explore regenerative medicine possibilities,' Professor Muzlifah Haniffa added. 'We are making exciting strides towards creating the Human Cell Atlas, understanding the biological steps of how humans are built, and investigating what goes wrong in disease.'
Dr Hudaa Gopee, co-first author, also added: ‘We're excited to have uncovered new important roles of immune cells during skin development that we could translate to the skin organoid model. These immune cells, called macrophages, promote the growth of skin blood vessels and also appear to play a key part in scarless skin repair. These findings could help improve other organoid models in the future and potentially inform clinical advances to avoid scarring after surgery.’
It is hoped that this information could be used in the creation of new hair follicles for regenerative medicine, such as for skin transplants for burn victims, or those with scarring alopecia.
You might also like:
- read the study: A human prenatal skin cell atlas reveals immune cell regulation of skin morphogenesis. Nusayhah Hudaa Gopee et al. Nature. DOI: 10.1038/s41586-024-08002-x
- learn more about the researchers involved in this study:
- Professor Muzlifah Haniffa, Wellcome Trust Senior Research Fellow in Clinical Science, Lister Institute Prize Fellow, Professor of Dermatology and Immunology, Newcastle University
- Dr Elena Winheim, Postdoctoral Fellow, Wellcome Trust Sanger Institute
- Dr Hudaa Gopee, Clinical Research Fellow, Newcastle University
- visit the Human Cell Atlas website or explore Nature's collection of research articles and related content
- find out more about our Ageing and Health research at Newcastle University
