How 3D structures of enzymes reveal how they work and what this means for rare disease therapies
24 November 2022 | By: Newcastle University | 2 min read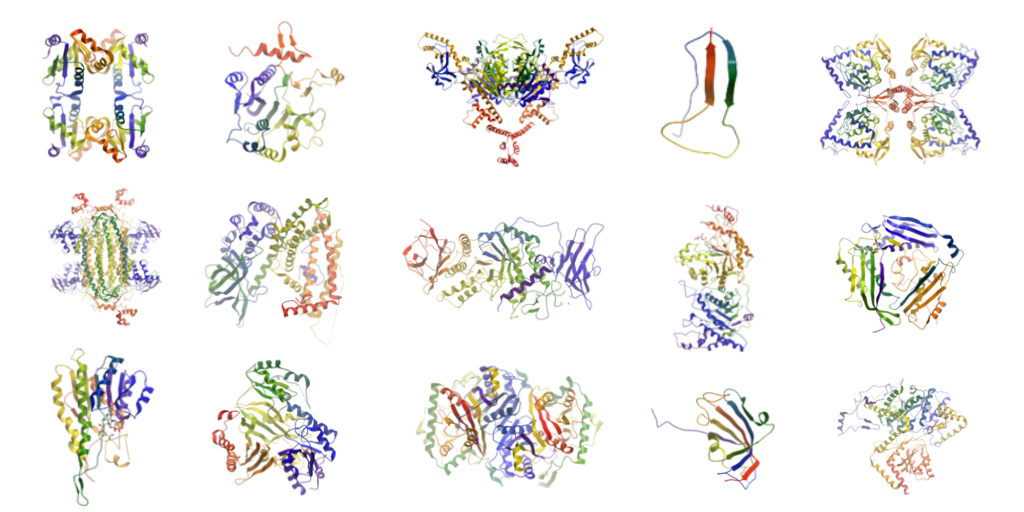
There are over 7000 recognised rare diseases but only around 5% have an approved treatment available. Professor Wyatt Yue’s team at our Biosciences Institute are exploring how the unique 3D structure of enzymes can help to develop therapies for rare diseases.
Rare diseases: the stumbling block
There are over 7000 recognised rare diseases but only around 5% have an approved treatment available. Most of those treatments are not transformative, but instead focus on managing the symptoms.
A major stumbling block in developing therapies is that most rare diseases are caused by genetic mutations that reduce or abolish an essential biological function. In contrast, many common diseases are the consequence of an increased or over-active biological function.
Blockages on the body’s conveyer belt
The team – which includes Prof Wyatt Yue, Dr Sabrina Mackinnon, and Dr Thomas McCorvie among others – is based at Newcastle University’s Centre for Rare Disease investigates a group of rare diseases that means someone’s metabolism does not function correctly and this is because an enzyme, the key protein worker within it, is broken.
Imagine metabolism as a factory. The protein worker is an essential part of a conveyor belt helping the factory to function. When this protein breaks, it causes a blockage in that conveyor belt which causes issues across the whole factory. Materials before the hold-up will build up and can lead to toxicity in the body, and materials that should travel along the conveyer belt after the hold-up are missing and can impact on essential body function.
Structures are key to determining function, dysfunction, and treatment
To develop new therapies for rare diseases such as these, three key pieces of information are needed:
- how the disease-causing mutations affect the enzyme causing the hold-up
- which part of the conveyor belt should we address to mitigate the hold-up
- what drug molecules we should use to do so
The team who are structural biologists believe that the 3D structure of an enzyme dictates how the enzyme works, and why it is not working when it breaks.
This would mean that it’s possible to determine what the enzyme should look like when functioning well and compare it to how the enzyme looks when it is broken. The 3D structure of an enzyme also guides the design of drug molecules that can act on it.
The image at the top of this blog shows some of the 60 metabolic enzymes that the Yue Lab have focussed on over the years to determine their 3D structures.
Exploring structures with cutting-edge technology
The team uses two cutting-edge technologies to look at structures.
In x-ray crystallography, many molecules of the enzyme are packed inside a crystal. An x-ray then passes through, revealing information about the atoms and bonds that make up the enzyme’s shape.
Cryo-electron microscopy is particularly suited for larger, more complex enzymes. Here, multiple photographs of the enzyme in a native-like environment are taken by the electron microscope and its overall shape is derived from averaging these photos.
These technologies expose – at the level of atoms and bonds – how an enzyme takes the shape that reflects its function. They also reveal what parts of the enzyme are important for working with other enzymes and materials, how the structure changes under disease conditions, and how the enzyme interacts with drug molecules.
Two methods to fix the conveyor belt
Once the structure is understood, the team can begin to develop therapies addressing two different aspects of the conveyor belt.
One approach aims to directly bolster the function of the broken enzyme. This can be done by designing drug molecules that hold the enzyme together so that it can resume work (partially). The team is taking on this approach to develop novel treatments for homocystinuria, a rare but potentially serious inherited condition where the body isn’t able to process methionine, the amino acid which is necessary in our diets for building protein.
The other approach aims to slow down the work carried out by another enzyme appearing before the broken enzyme in the conveyor belt.
This would mean that the overall flow of materials in the conveyor belt minimises any hold-up in the process. The team is exploring this route in order to design new treatment for an epileptic disorder and a kidney disease.
Turning the tide for rare diseases in the future
Professor Yue’s team enjoys an active role in the University’s rare disease ecosystem and drug discovery research community.
It is the team’s vision that, as cutting-edge technologies – such as those developed to study enzyme shapes – advance at an unprecedented rate, researchers can turn the tide in rare disease therapy by helping to make the drug discovery process quicker, easier and cheaper.

Photo of the Yue Lab, taken on Rare Disease Day 2022. Each member holds up the name of the rare disease that their research impacts on.
As a centre of research excellence, Newcastle has a long and distinguished history of research and clinical care in rare disease.
Discover more about how we improve therapy options, outcomes, and quality of life for people with a rare disease.
